Categories: Featured Articles » Interesting electrical news
Number of views: 51203
Comments on the article: 7
What are fuel cells
 Mobile electronics every year, if not a month, is becoming more accessible and widespread. Here you have laptops, and PDAs, and digital cameras, and mobile phones, and a ton of all sorts of useful and not so devices. And all these devices are continuously acquiring new features, more powerful processors, large color screens, wireless communications, while at the same time decreasing in size. But, unlike semiconductor technologies, the power technologies of all this mobile menagerie do not go by leaps and bounds.
Mobile electronics every year, if not a month, is becoming more accessible and widespread. Here you have laptops, and PDAs, and digital cameras, and mobile phones, and a ton of all sorts of useful and not so devices. And all these devices are continuously acquiring new features, more powerful processors, large color screens, wireless communications, while at the same time decreasing in size. But, unlike semiconductor technologies, the power technologies of all this mobile menagerie do not go by leaps and bounds.
Conventional rechargeable batteries and batteries are clearly not enough to power the latest advances in the electronics industry for any substantial amount of time. And without reliable and capacious batteries, the whole point of mobility and wirelessness is lost. So the computer industry is working more and more actively on the problem alternative power supplies. And the most promising, today, direction is fuel cells.
The basic principle of operation of fuel cells was discovered by British scientist Sir William Grove in 1839. He is known as the father of the fuel cell. William Grove generated electricity by changing electrolysis of water to extract hydrogen and oxygen. Having disconnected the battery from the electrolytic cell, Grove was surprised to find that the electrodes began to absorb the released gas and generate current. Process opening electrochemical cold combustion of hydrogen an event in the energy sector became significant, and in the future such well-known electrochemists as Ostwald and Nernst played a large role in the development of the theoretical foundations and practical implementation of fuel cells and predicted a great future for them.
Himself the term "fuel cell" (Fuel Cell) appeared later - it was proposed in 1889 by Ludwig Mond and Charles Langer, who tried to create a device for generating electricity from air and coal gas.
During normal combustion, oxygen oxidizes fossil fuels, and the chemical energy of the fuel is inefficiently converted into heat. But it turned out to be possible to carry out the oxidation reaction, for example, hydrogen with oxygen, in an electrolyte medium and in the presence of electrodes to obtain an electric current. For example, supplying hydrogen to an electrode located in an alkaline medium, we obtain electrons:
2H2 + 4OH- → 4H2O + 4e-
which, passing through an external circuit, go to the opposite electrode, to which oxygen enters and where the reaction takes place: 4e- + O2 + 2H2O → 4OH-
It can be seen that the resulting reaction 2H2 + O2 → H2O is the same as during normal combustion, but in the fuel cell, or otherwise, in electrochemical generator, it turns out electric current with high efficiency and partially heat. Note that coal, carbon monoxide, alcohols, hydrazine, other organic substances can also be used as fuel in fuel cells, and air, hydrogen peroxide, chlorine, bromine, nitric acid, etc. can be used as oxidizing agents.
The development of fuel cells continued vigorously both abroad and in Russia, and further in the USSR. Among the scientists who made a great contribution to the study of fuel cells, we mention V. Zhako, P. Yablochkov, F. Bacon, E. Bauer, E. Justi, K. Cordes. In the middle of the last century, a new assault on fuel cell problems began. This is partly due to the emergence of new ideas, materials and technologies as a result of defense research.
One of the scientists who made a major step in the development of fuel cells was P. M. Spiridonov. Hydrogen-oxygen elements of Spiridonov gave a current density of 30 mA / cm2, which for that time was considered a great achievement.In the forties, O. Davtyan created an installation for the electrochemical burning of generator gas obtained by coal gasification. With each cubic meter of element volume, Davtyan received 5 kW of power.
It was first solid electrolyte fuel cell. It had high efficiency, but over time, the electrolyte became unusable, and it had to be changed. Subsequently, Davtyan in the late fifties created a powerful installation that drives the tractor. In those same years, the English engineer T. Bacon designed and built a fuel cell battery with a total capacity of 6 kW and an efficiency of 80%, operating on pure hydrogen and oxygen, but the ratio of power to battery weight was too small - such elements were unsuitable for practical use and too dear ones.
In subsequent years, the time of loners has passed. The creators of spacecraft became interested in fuel cells. Since the mid-60s, millions of dollars have been invested in fuel cell research. The work of thousands of scientists and engineers allowed to reach a new level, and in 1965. fuel cells were tested in the United States on the Gemini 5 spaceship, and later on on Apollo ships for flights to the moon and under the Shuttle program.
In the USSR, fuel cells were developed at NPO Kvant, also for use in space. In those years, new materials already appeared - solid polymer electrolytes based on ion-exchange membranes, new types of catalysts, electrodes. And yet, the working current density was small - in the range of 100-200 mA / cm2, and the platinum content on the electrodes was several g / cm2. There were many problems associated with durability, stability, and safety.
The next stage of rapid development of fuel cells began in the 90s. last century and continues now. It is caused by the need for new efficient energy sources due, on the one hand, to the global environmental problem of the increasing greenhouse gas emissions from the combustion of fossil fuels and, on the other hand, to the depletion of such fuels. Since water is the end product of hydrogen combustion in a fuel cell, they are considered the cleanest from the point of view of environmental impact. The main problem is only to find an effective and inexpensive way to produce hydrogen.
Billion financial investments in the development of fuel cells and hydrogen generators should lead to a technological breakthrough and make their use in everyday life a reality: in cell phone cells, in cars, in power plants. Already, automobile giants such as Ballard, Honda, Daimler Chrysler, General Motors are demonstrating cars and buses running on 50kW fuel cells. A number of companies have developed solid fuel electrolyte fuel cell demonstration plants up to 500 kW. But, despite a significant breakthrough in improving the performance of fuel cells, many problems still need to be solved related to their cost, reliability, and safety.
In a fuel cell, unlike batteries and accumulators, both fuel and oxidizer are supplied from outside. The fuel cell is only a mediator in the reaction and under ideal conditions could work almost forever. The beauty of this technology is that in fact, fuel is burned in the element and the energy released is directly converted into electricity. With direct combustion of fuel, it is oxidized with oxygen, and the heat generated in this process is used to complete useful work.
In a fuel cell, as in batteries, the reactions of fuel oxidation and oxygen reduction are spatially separated, and the process of "burning" proceeds only if the cell gives off current to the load. It's like diesel generator, only without diesel and generator. And also without smoke, noise, overheating and with much higher efficiency. The latter is explained by the fact that, firstly, there are no intermediate mechanical devices and, secondly, the fuel cell is not a heat engine and therefore does not obey the Carnot law (that is, its efficiency is not determined by the temperature difference).
Oxygen is used as an oxidizing agent in fuel cells. Moreover, since oxygen is quite enough in the air, there is no need to worry about the supply of an oxidizing agent. As for fuel, it is hydrogen. So, the reaction occurs in the fuel cell:
2H2 + O2 → 2H2O + electricity + heat.
The result is useful energy and water vapor. The simplest in its design is proton exchange membrane fuel cell (see figure 1). It works as follows: the hydrogen entering the element decomposes under the action of the catalyst into electrons and positively charged hydrogen ions H +. Then a special membrane comes into play, acting here as an electrolyte in a conventional battery. Due to its chemical composition, it passes protons through itself, but it traps electrons. Thus, the electrons accumulated on the anode create an excess negative charge, and hydrogen ions create a positive charge on the cathode (the voltage on the element is about 1V).
To create high power, a fuel cell is assembled from a plurality of cells. If you include an element in the load, then the electrons flow through it to the cathode, creating a current and completing the process of hydrogen oxidation by oxygen. As a catalyst in such fuel cells, platinum microparticles deposited on a carbon fiber are typically used. Due to its structure, such a catalyst passes gas and electricity well. The membrane is typically made from a sulfur-containing Nafion polymer. The thickness of the membrane is equal to tenths of a millimeter. During the reaction, of course, heat is also released, but it is not so much, so that the operating temperature is maintained in the range of 40-80 ° C.
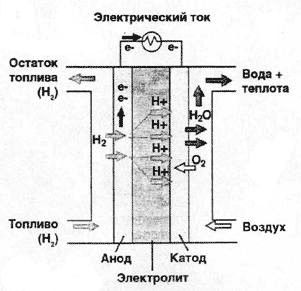
Fig. 1. The principle of operation of the fuel cell
There are other types of fuel cells, mainly differing in the type of electrolyte used. Almost all of them require hydrogen as a fuel, so a logical question arises: where to get it. Of course, it would be possible to use compressed hydrogen from cylinders, but then immediately problems arise associated with the transportation and storage of this highly flammable gas under high pressure. Of course, you can use hydrogen in bound form as in metal hydride batteries. But still, the task remains of its extraction and transportation, because the infrastructure of hydrogen gas stations does not exist.
However, there is also a solution - liquid hydrocarbon fuel can be used as a source of hydrogen. For example, ethyl or methyl alcohol. True, a special additional device is already required here - a fuel converter, which converts alcohols into a mixture of gaseous H2 and CO2 at high temperature (for methanol it will be somewhere around 240 ° C). But in this case it is already more difficult to think about portability - such devices are well used as stationary or car alternatorsBut for compact mobile equipment you need something less cumbersome.
And here we come to that device, the development of which almost all the largest manufacturers of electronics are engaged with terrible force - methanol fuel cell (figure 2).
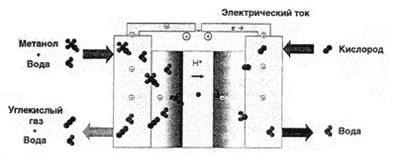
Fig. 2. The principle of operation of the fuel cell on methanol
The fundamental difference between hydrogen and methanol filling elements is the catalyst used. The catalyst in the methanol fuel cell allows protons to be removed directly from the alcohol molecule.Thus, the issue of fuel is being solved - methyl alcohol is mass-produced for the chemical industry, it is easy to store and transport, and to charge a methanol fuel cell, it is enough to simply replace the cartridge with fuel. True, there is one significant minus - methanol is toxic. In addition, the efficiency of a methanol fuel cell is significantly lower than that of a hydrogen one.

Fig. 3. Methanol fuel cell
The most tempting option is to use ethyl alcohol as a fuel, since the production and distribution of alcoholic beverages of any composition and strength is well established around the globe. However, the efficiency of ethanol fuel cells, unfortunately, is even lower than that of methanol.
As already noted over many years of development in the field of fuel cells, various types of fuel cells have been built. Fuel cells are classified by electrolyte and type of fuel.
1. Solid polymer hydrogen-oxygen electrolyte.
2. Solid polymer methanol fuel cells.
3. Elements on alkaline electrolyte.
4. Phosphoric acid fuel cells.
5. Fuel cells on molten carbonates.
6. Solid oxide fuel cells.
Ideally, the efficiency of fuel cells is very high, but in real conditions there are losses associated with nonequilibrium processes, such as ohmic losses due to the conductivity of the electrolyte and electrodes, activation and concentration polarization, diffusion losses. As a result of this, part of the energy generated in the fuel cells is converted into heat. The efforts of specialists are aimed at reducing these losses.
The main source of ohmic losses, as well as the reason for the high price of fuel cells, are perfluorinated sulfocation-ion exchange membranes. Now we are looking for alternative, cheaper proton-conducting polymers. Since the conductivity of these membranes (solid electrolytes) reaches an acceptable value (10 Ohm / cm) only in the presence of water, the gases supplied to the fuel cell must be additionally moistened in a special device, which also makes the system more expensive. In catalytic gas diffusion electrodes, mainly platinum and some other noble metals are used, and so far no replacement has been found. Although the platinum content in the fuel cells is several mg / cm2, for large batteries its amount reaches tens of grams.
When designing fuel cells, much attention is paid to the heat removal system, since at high current densities (up to 1A / cm2) the system self-heats up. For cooling, water circulating in the fuel cell through special channels is used, and at low capacities, air blowing is used.
So, the modern system of the electrochemical generator, besides the fuel cell battery itself, “grows” with many auxiliary devices, such as: pumps, compressor for air supply, hydrogen inlet, gas humidifier, cooling unit, gas leakage control system, DC to AC converter, control processor etc. All this leads to the fact that the cost of the fuel cell system in 2004-2005 was 2-3 thousand $ / kW. According to experts, fuel cells will become available for use in transport and in stationary power plants at a price of 50-100 $ / kW.
For the introduction of fuel cells in everyday life, along with the cheaper components, you need to expect new original ideas and approaches. In particular, high hopes are associated with the use of nanomaterials and nanotechnology. For example, recently several companies announced the creation of super-efficient catalysts, in particular, for an oxygen electrode based on clusters of nanoparticles of various metals. In addition, there have been reports of the design of membrane-free fuel cells in which liquid fuel (e.g. methanol) is supplied to the fuel cell along with an oxidizing agent.An interesting concept is also the developed concept of biofuel elements operating in polluted waters and consuming dissolved oxygen as an oxidizing agent, and organic impurities as fuel.
According to experts, fuel cells will enter the mass market in the coming years. Indeed, developers conquer technical problems one after another, report on successes and present prototypes of fuel cells. For example, Toshiba has demonstrated a finished prototype methanol fuel cell. It has a size of 22x56x4.5mm and gives a power of the order of 100mW. One refueling in 2 cubes of concentrated (99.5%) methanol is enough for 20 hours of work of the MP3 player. Toshiba has released a commercial fuel cell to power mobile phones. Again, the same Toshiba demonstrated an element for powering laptops measuring 275x75x40mm, enabling the computer to work for 5 hours from a single refueling.
Another Japanese company, Fujitsu, is not far behind Toshiba. In 2004, she also introduced an element acting on a 30% aqueous solution of methanol. This fuel cell worked at one refueling in 300 ml for 10 hours and at the same time gave out power of 15 watts.
Casio is developing a fuel cell in which methanol is first processed into a mixture of gaseous H2 and CO2 in a miniature fuel converter, and then fed into the fuel cell. During the demonstration, the Casio prototype provided power to the laptop for 20 hours.
Samsung was also noted in the field of fuel cells - in 2004, it demonstrated its 12 W prototype designed to power a laptop. In general, Samsung expects to use fuel cells, primarily in fourth-generation smartphones.
I must say that Japanese companies generally very thoroughly approached the development of fuel cells. Back in 2003, companies such as Canon, Casio, Fujitsu, Hitachi, Sanyo, Sharp, Sony and Toshiba joined forces to develop a common fuel cell standard for laptops, mobile phones, PDAs and other electronic devices. American companies, which are also numerous in this market, mostly work under contracts with the military and develop fuel cells for electrifying American soldiers.
The Germans are not far behind - Smart Fuel Cell sells fuel cells to power a mobile office. The device is called Smart Fuel Cell C25, it has dimensions of 150x112x65mm and can produce up to 140 watts-hours at a single gas station. This is enough to power the laptop for about 7 hours. Then the cartridge can be replaced and you can continue to work. The size of the cartridge with methanol is 99x63x27 mm, and it weighs 150g. The system itself weighs 1.1 kg, so you can’t call it portable at all, but it’s still a complete and convenient device. The company is also developing a fuel module for powering professional video cameras.
In general, fuel cells have almost entered the mobile electronics market. Manufacturers are left to solve the latest technical problems before starting mass production.
Firstly, it is necessary to solve the issue of miniaturization of fuel cells. After all, the smaller the fuel cell, the less power it will be able to give out - so new catalysts and electrodes are constantly being developed that allow, at small sizes, to maximize the working surface. Here, just in time, the latest developments in the field of nanotechnology and nanomaterials (for example, nanotubes) come in handy. Again, to miniaturize the piping of elements (fuel and water pumps, cooling and fuel conversion systems), microelectromechanical advances are increasingly being applied.
The second important problem that needs to be addressed is the price. Indeed, as a catalyst in most fuel cells, very expensive platinum is used.Again, some of the manufacturers are trying to make the most of the already well-developed silicon technology.
As for other areas of the use of fuel cells, the fuel cells are already well established there, although they have not yet become mainstream in the energy sector or in transport. Already, many car manufacturers have presented their fuel cell powered concept cars. In several cities around the world, fuel cell buses are running around. Canadian Ballard Power Systems produces a range of stationary generators ranging from 1 to 250 kW. At the same time, kilowatt generators are designed to immediately supply one apartment with electricity, heat and hot water.
See also: Alternative energy sources
See also at bgv.electricianexp.com
:
