Categories: Novice electricians, Industrial electrician
Number of views: 37528
Comments on the article: 0
The practical application of electrolysis
When an electric current passes through a solution or a melt of an electrolyte, dissolved electrodes or other substances that are the products of secondary reactions on the electrodes are released on the electrodes. This physicochemical process is called electrolysis.
The essence of electrolysis
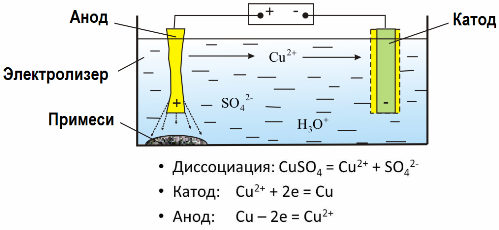
In the electric field created by the electrodes, the ions in the conducting fluid come in an ordered motion. The negative electrode is the cathode, the positive is the anode.
Negative ions, called anions (ions of the hydroxyl group and acid residues) rush to the anode, and positive ions, called cations (hydrogen, metal, ammonium ions, etc.) rush to the cathode
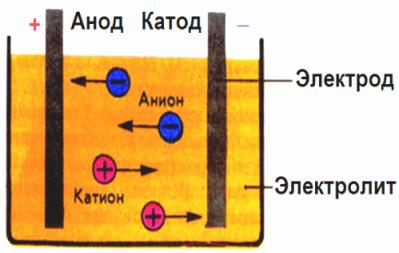
An oxidation-reduction process takes place at the electrodes: electrochemical reduction of particles (atoms, molecules, cations) occurs at the cathode, and electrochemical oxidation of particles (atoms, molecules, anions) occurs at the anode. Dissociation reactions in an electrolyte are primary reactions, and reactions that proceed directly at the electrodes are called secondary.
The laws of Faraday electrolysis
The separation of electrolysis reactions into primary and secondary helped Michael Faraday establish the laws of electrolysis:
-
The first law of Faraday electrolysis: the mass of the substance deposited on the electrode during electrolysis is directly proportional to the amount of electricity transferred to this electrode. By the amount of electricity is meant an electric charge, measured, as a rule, in pendants.
-
The second law of Faraday electrolysis: for a given amount of electricity (electric charge), the mass of a chemical element deposited on the electrode is directly proportional to the equivalent mass of the element. The equivalent mass of a substance is its molar mass divided by an integer, depending on the chemical reaction in which the substance is involved.
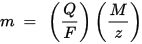
m is the mass of the substance deposited on the electrode, Q is the total electric charge passing through the substance F = 96,485.33 (83) C mol – 1 is the Faraday constant, M is the molar mass of the substance (For example, the molar mass of water H2O = 18 g / mol), z is the valence number of ions of a substance (the number of electrons per ion).
Note that M / z is the equivalent mass of the precipitated substance. For the first Faraday law, M, F and z are constants, so the larger the value of Q, the greater the value of m. For the second Faraday law, Q, F and z are constants, so the larger the value of M / z (equivalent mass), the greater the value of m.
Electrolysis is widely used today in industry and in technology. For example, it is electrolysis that serves as one of the most effective methods for the industrial production of hydrogen, hydrogen peroxide, manganese dioxide, aluminum, sodium, magnesium, calcium and other substances. Electrolysis is used to treat wastewater, in electroplating, in electroplating, and finally in chemical current sources. But first things first.
Obtaining pure metals from ores by electrolysis
Thanks to electrolysis, many metals are extracted from ores and subjected to further processing. So, when ore or enriched ore — concentrate — is treated with reagents, the metal passes into the solution, and then, by electroextraction, the metal is isolated from the solution. Pure metal is released at the same time at the cathode. In this way receive zinc, copper, cadmium.
Metals are subjected to electrorefining to eliminate impurities and to convert the contained impurities into a form convenient for further processing. The metal to be cleaned is cast in the form of plates, and these plates are used as anodes in electrolysis.
When the current passes, the metal of the anode dissolves, passes in the form of cations into the solution, then the cations are discharged at the cathode and form a precipitate of pure metal. The impurities of the anode do not dissolve - they precipitate with the anode slurry, or pass into the electrolyte, from where they are continuously or periodically removed.
Consider as an example copper electrorefining. The main component of the solution - copper sulfate - the most common and cheapest salt of this metal. The solution has a low electrical conductivity. To increase it, sulfuric acid is added to the electrolyte.
In addition, small amounts of additives are introduced into the solution to facilitate the formation of a compact metal precipitate. In general, copper, nickel, lead, tin, silver, and gold are subjected to electrolytic refining.
Electrolysis Wastewater Treatment
Electrolysis is used in wastewater treatment (processes of electrocoagulation, electroextraction and electroflotation). The electrochemical cleaning method is one of the most commonly used. For electrolysis, insoluble anodes (magnetite, lead oxide, graphite, manganese, which are deposited on a titanium base), or soluble (aluminum, iron) are used.
This method is used to isolate toxic organic and inorganic substances from water. For example, copper pipes are cleaned of scale with a solution of sulfuric acid, and industrial wastewater must then be cleaned by electrolysis with an insoluble anode. Copper is released at the cathode, which can again be used in the same enterprise.
Alkaline wastewater is purified by electrolysis from cyanide compounds. In order to accelerate the oxidation of cyanides, increase electrical conductivity and save energy, an additive in the form of sodium chloride is used in water.
Electrolysis is carried out with a graphite anode and a steel cathode. Cyanides are destroyed during electrochemical oxidation and chlorine, which is released on the anode. The effectiveness of such cleaning is close to 100%.
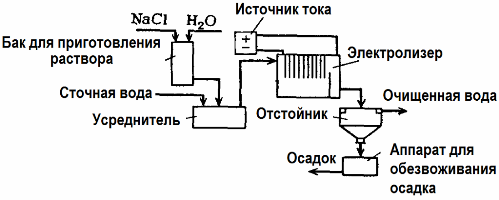
In addition to direct electrochemical cleaning, it can be included in the electrolysis process coagulation. Excluding the addition of salts, electrolysis is carried out with soluble aluminum or iron anodes. Then not only the contaminants on the anode are destroyed, but the anode itself dissolves. Active dispersed compounds are formed that coagulate (thicken) colloidal dispersed contaminants.
This method is effective in the treatment of wastewater from fats, oil products, dyes, oils, radioactive substances, etc. It is called electrocoagulation.
Electroplating
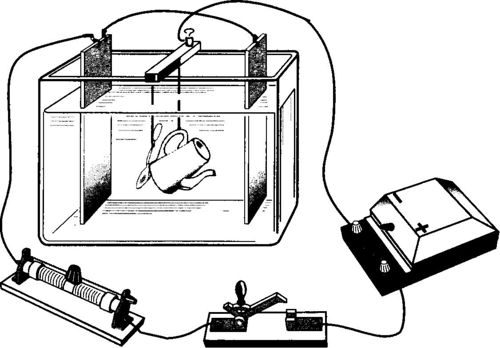
Electroplating is the electrolytic deposition of certain metals in order to protect products from corrosion and to give them an appropriate aesthetic appearance (coating is made with chromium, nickel, silver, gold, platinum, etc.). The thing is thoroughly cleaned, degreased, and used as a cathode in an electrolytic bath in which a salt solution of the metal with which it is necessary to coat the product is poured.
A plate of the same metal is used as the anode. As a rule, a pair of anode plates is used, and the subject to be galvanized is placed between them.
Electrotype
Electroplating - the deposition of metal on the surface of different bodies to reproduce their shapes: molds for casting parts, sculptures, printed cliches, etc.

Galvanic deposition of metal on the surface of an object is possible only when this surface or the entire object is an electric current conductor, so it is advisable to use metals to make models or forms. Fusible metals are most suitable for this purpose: lead, tin, solders, Wood alloy.
These metals are soft, easily processed with metalwork tools, are well engraved and cast. After building up the galvanic layer and finishing, the mold metal is smelted from the finished product.
However, the greatest opportunities for the manufacture of models are still represented by dielectric materials. To metallize such models, it is necessary to give their surface electrical conductivity. Success or failure ultimately depends mainly on the quality of the conductive layer. This layer can be applied in one of three ways.
The most common way is graphitization, it is suitable for models of plasticine and other materials that allow grinding of graphite on the surface.
The next trick is bronzing, the method is good for models of relatively complex shape, for different materials, however, due to the thickness of the bronze layer, the transfer of small parts is somewhat distorted.
And finally silveringsuitable in all cases, but especially indispensable for fragile models with a very complex shape - plants, insects, etc.
Chemical current sources
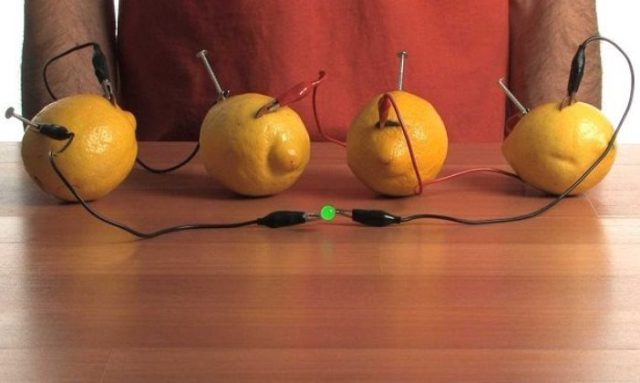
Also, electrolysis is the main process due to which the most advanced chemical current sources, such as batteries and accumulators, function. There are two electrodes in contact with the electrolyte.
A potential difference is established between the electrodes - an electromotive force corresponding to the free energy of the redox reaction. See here for more details: Chemical sources of electric current
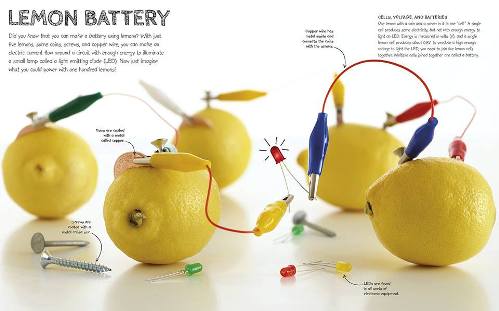
Lemon battery (click on the picture to enlarge)
The action of chemical current sources is based on the occurrence of spatially separated processes with a closed external circuit: on the negative anode, the reducing agent is oxidized, the free electrons that are formed pass through the external circuit to the positive cathode, creating a discharge current, where they participate in the oxidation reduction reaction. Thus, the flow of negatively charged electrons along the external circuit goes from the anode to the cathode, that is, from the negative electrode to the positive one.
See also at bgv.electricianexp.com
: