Categories: Featured Articles » Interesting Facts
Number of views: 13392
Comments on the article: 2
Aluminum is more expensive than gold
 Did you know that the possession of any aluminum product, such as a profile, a sleeve, a spoon or an element of accessories, in the 19th century would already have made you quite a wealthy person? Today, of course, it is well known that aluminum is very common around the world, but before it was valued more than gold. But the thing is that there is no aluminum in pure metal form in the earth’s crust, although in the form of chemical compounds it makes up almost 8% of the earth’s crust.
Did you know that the possession of any aluminum product, such as a profile, a sleeve, a spoon or an element of accessories, in the 19th century would already have made you quite a wealthy person? Today, of course, it is well known that aluminum is very common around the world, but before it was valued more than gold. But the thing is that there is no aluminum in pure metal form in the earth’s crust, although in the form of chemical compounds it makes up almost 8% of the earth’s crust.
In ancient times, double aluminum salts (then they were not called so) - alum - were widely used to solve various problems, although aluminum was not discussed as such. The trivalent metal present in the salts allowed the use of alum for various purposes, and even today alum is used in antibacterial soap, in after-shave lotions, in baking powder.
Alumium-potassium alum was widely used in ancient times as a mordant and as a means of stopping bleeding. A solution of alum-potassium alum was impregnated with wood, which made it non-combustible. A well-known historical story testifies to how the Roman commander Archelaus, during the time of the war with the Persians, ordered to smear the towers of the defensive structures with alum, due to which the Persians, with all desire, could not set fire to them, not just to burn them.
 It was only in 1807 that the English chemist, physicist and geologist, Sir Humphry Davy, began to speak seriously about aluminum contained in alum, and he noted that, in addition to salts, some metal was also present in alum. Humphrey Davy decided to call this metal “aluminum”, since the word “alum” in Latin is alum.
It was only in 1807 that the English chemist, physicist and geologist, Sir Humphry Davy, began to speak seriously about aluminum contained in alum, and he noted that, in addition to salts, some metal was also present in alum. Humphrey Davy decided to call this metal “aluminum”, since the word “alum” in Latin is alum.
In fairness, it is worth mentioning that in France, 29 years before Davy, the chemist Antoine Lavoisier already pointed out in his chemistry works on alumina, which he called “agril”, and at the same time noted that this substance, probably can exist in solid form, that is, in the form of metal. Although technologically in those years it was still impossible to separate strong oxygen atoms from oxide molecules.
The first major success came in 1825 when a Danish physicist and electromagnetist, Hans Christian Oersted, in his laboratory heated anhydrous aluminum chloride (obtained by passing chlorine through a red-hot mixture of aluminum oxide and coal) with potassium amalgam, and, having driven away the mercury, got aluminum , although slightly contaminated with impurities, confirming, however, thereby Davy's fundamentally important idea.
In honor of a colleague of the Englishman who inspired Oersted to conduct this experiment, Oersted called the metal obtained aluminum. Oersted is now considered the first scientist who received aluminum in the laboratory.
Two years after the experiment, Oersted, a German physicist and medical doctor, Friedrich Wöhler, developed a new laboratory method for the production of aluminum, improving the Oersted method. Wöhler was able to obtain aluminum in the form of a powder of granules, as a result of heating aluminum chloride with potassium. In a similar way, Wöhler then received beryllium and yttrium.
Over the next 18 years, until 1845, scientists have already produced enough metal to study its properties in detail. But it was Weller who noted the unusual lightness of aluminum, compared with other metals.
Nine years later, namely, in 1854, the French physicist and chemist Henri Saint-Clair Deville managed to develop a much more practical method for producing aluminum. He used metallic sodium to displace aluminum from double sodium chloride and aluminum. It was a method by which it was possible to obtain several kilograms of pure aluminum at a time. Two years later, Henri St. Clair Deville will be the first to obtain aluminum by electrolysis of molten sodium chloride-aluminum.
An interesting historical fact.In 1855, Napoleon III organized an exhibition of aluminum ingots. 12 miniature ingots impressed the guests of the exhibition with their brilliance, while being very light.
So aluminum has become an ideal metal for the production of jewelry and various items of clothing, such as, for example, buckles, and for a long time was not the last of the museum exhibits. This fact infuriated Henri - the value of aluminum should not be limited to trinkets.
The emperor, who sponsored the researcher in his work, hoped that weapons and armor could be made of aluminum, and even several helmets were made, and as a result, there was a disappointment in the properties of the metal. Napoleon III ordered the processing of all the aluminum obtained for the production of cutlery.

These cutlery was used only by higher persons, including the emperor himself, while guests were given only golden spoons and forks. In those days, aluminum was harder to obtain than gold, and its price was therefore many times higher than gold.
In 1886, the situation changed. The method of industrial production of aluminum was discovered by electrolysis. The simultaneous discovery, independently of each other, was made by the French chemical engineer Paul-Louis-Toussin Eru and the American Charles Martin Hall, also a chemical engineer. It is known that Hall was at first very surprised when he discovered plaques of pure aluminum at the bottom of the vessel.
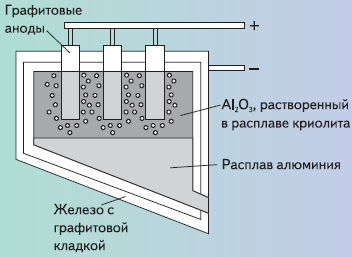
To this day, this method bears the name of its inventors - the Hall – Eru process — the dissolution of alumina in a cryolite melt, followed by electrolysis using consumable coke or graphite anode electrodes. In the 20th century, this method was used very widely for the industrial production of aluminum.
In general, just two years after the opening of Hall and Eru, a Russian chemist of Austrian origin, Karl Iosifovich Bayer, proposed cheaply producing aluminum oxide from bauxite to obtain aluminum oxide.
So the price of aluminum fell five times in one night. Ultimately, if in 1852 a kilogram of aluminum was worth $ 1,200, then by the beginning of the 20th century, a kilogram was already worth less than a dollar. And today, aluminum products are generally not very expensive.

The resulting metal was good for everyone except the strength so necessary in industry. But this problem was later resolved. In 1903, German metallurgical engineer Alfred Wilm found that aluminum alloy with the addition of 4% copper after quenching (quenching temperature 500 ° C), being at room temperature for 4-5 days, gradually becomes harder and stronger, without losing with plasticity.

In 1909, Wilm filed an application for a patent "Method for improving aluminum alloys containing magnesium." On an industrial scale, they began to obtain durable aluminum alloy in 1911 in the German town of Düren, in honor of which this alloy was called "duralumin."
See also at bgv.electricianexp.com
:
